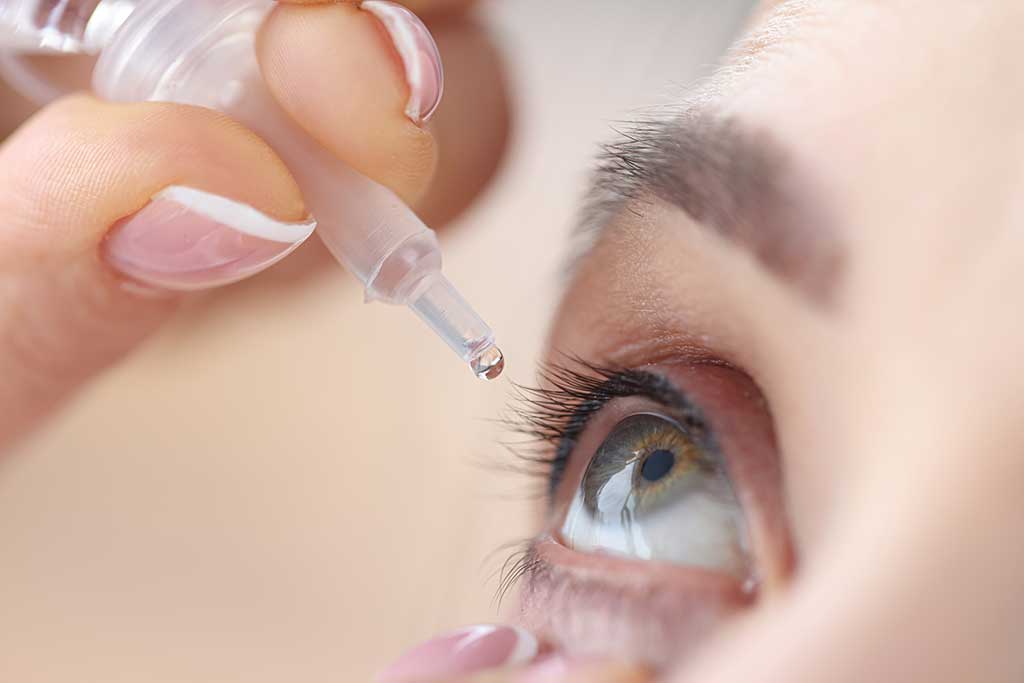
From 1 October 2021, ciclosporin is available on the Pharmaceutical Benefits Scheme (PBS) as an eye drop. This product, Ikervis®, is indicated for the treatment of dry eye disease with keratitis. Dry eye disease is a relatively common condition that can lead to chronic inflammation and damage of the ocular surface. These effects are thought to be largely mediated by T lymphocytes.
When administered systemically, ciclosporin is an immunosuppressant used to prevent transplant rejection and treat some autoimmune conditions. Although the exact mechanism of action in dry eye disease is not known, ciclosporin eye drops are thought to produce an immunomodulatory effect. In particular, ciclosporin inhibits the activation of T lymphocytes as well as other cell-mediated inflammatory pathways.
The SANSIKA study evaluated the safety and efficacy of ciclosporin for the treatment of dry eye disease with keratitis. This randomised, double-blind study assigned patients to a 0.1% ciclosporin emulsion or its vehicle for six months. A corneal fluorescein staining score improvement of at least three grades occurred in 31.2% of the ciclosporin group compared to 13.2% of the vehicle group at six months. The most commonly reported treatment-related adverse effect was instillation site pain. No systemic adverse effects were reported. However, to reduce systemic absorption, patients should be instructed to use nasolacrimal occlusion and close the eyes for two minutes after instillation.
References:
- Baudouin C, Sainz de la Maza M, Amrane M, Garrigue JS, Ismail D, Figueiredo FC, et al. One-year efficacy and safety of 0.1% cyclosporine a cationic emulsion in the treatment of severe dry eye disease. Eur J Ophthalmol. 2017; 27(6): 678-85.
- Department of Health. Australian Public Assessment Report for Ciclosporin. Woden: Therapeutic Goods Administration; 2021.
- Ikervis® (Ciclosporin) Australian approved product information. Parkville: Sequirus. Approved December 2020.
- Pflugfelder SC, de Paiva CS. The pathophysiology of dry eye disease: What we know and future directions for research. Ophthalmology 2017; 124 (11 Suppl): S4-S13.
Subscribe Knowledge Centre Updates
Enter your details to receive Knowledge Centre updates
